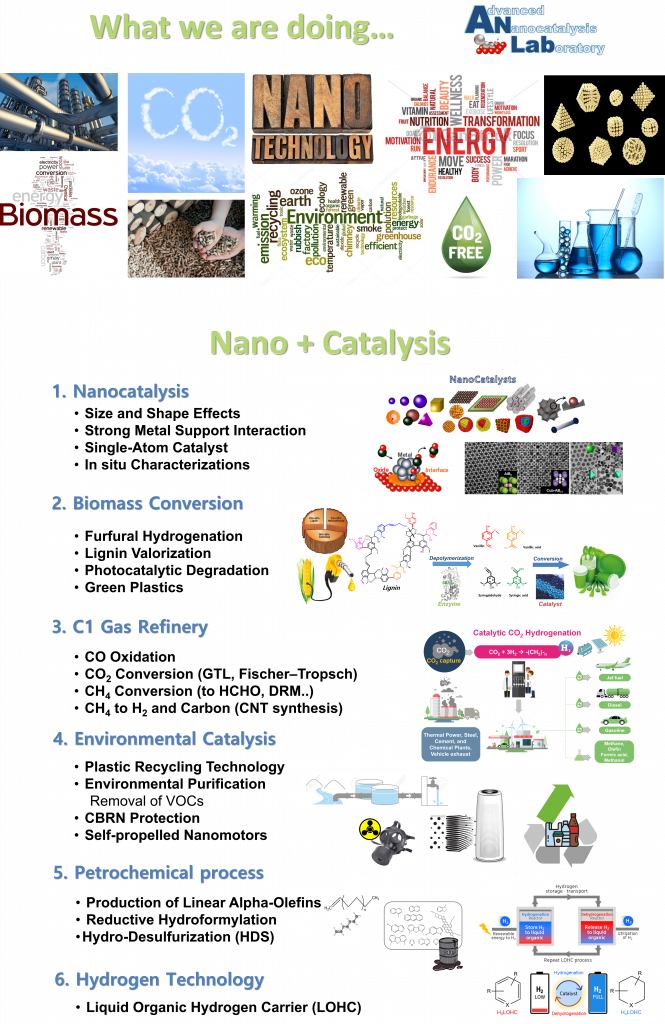
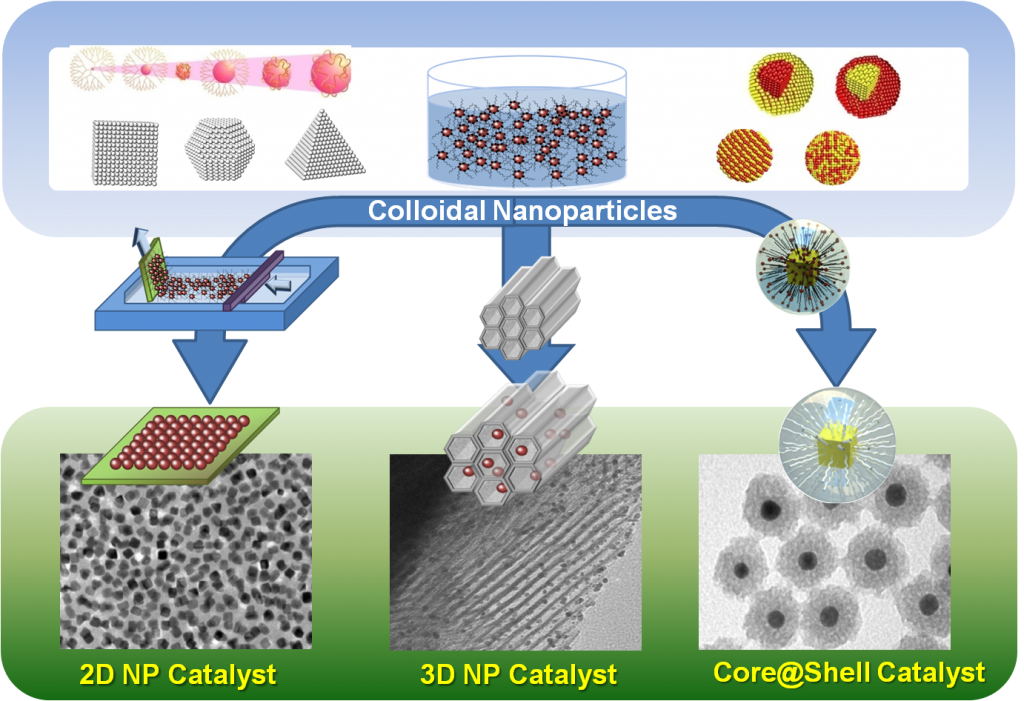
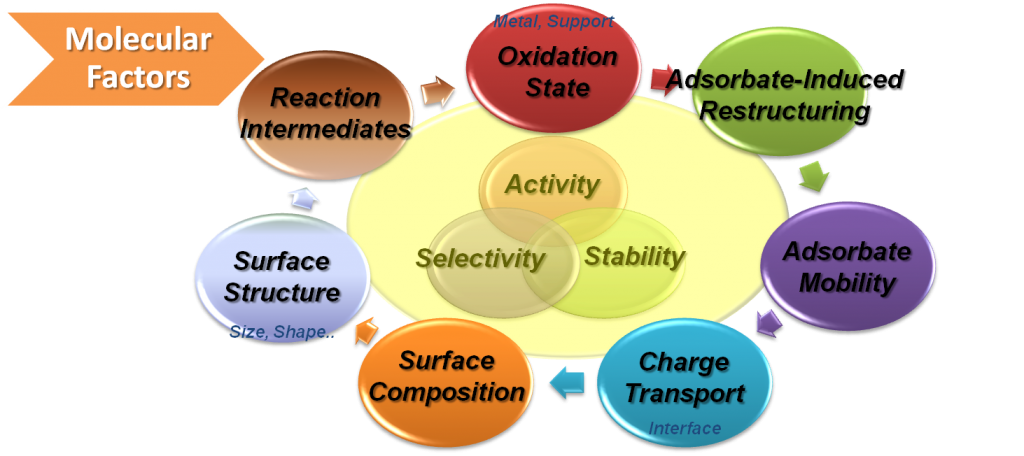
Based on current model reaction studies, it has been proven that turnover rates and selectivity of reactions were strongly influenced by the size, shape, and composition of nanoparticles and strong oxide-metal interactions, which are created by various metal-oxide interfaces.
With the evolution of in situ characterization techniques, we discovered that several molecular factors influenced catalytic activity and selectivity including surface structure and composition of nanoparticles, reaction intermediates, adsorbates, and oxidation states in nanocatalysis.
Especially, the interaction between the active metal and the oxide support induces charge transfer, resulting in enhancement of catalytic reaction rates and changing product selectivity.
These phenomena are called as the strong metal-support interaction (SMSI). Recent studies have demonstrated that electronic excitation in exothermic reactions involves the hot electron flow at oxide-metal interfaces.
When we utilize SMSI, catalytic performance can be improved beyond the intrincsic properties of metal or support. By maximizing the interfacial effect, we can develop new catalysts and catalytic systems, having outstanding performance with minmum consumption of novel metals.